Buba Blend
Well-Known Member
Went to a CVS pharmacy and bought the only type they had, their brand for $1.87. Went there because I never go there and hoped it would be different from my last gallon. Tested in a vial, a glass and a plastic cup. All three look like 6 to me. Tested my tap that is always 8 to check the drops and it looked like 8.
Had the pool drops avail so I checked with those since they are within range and it looked like 6 and again my tap looked like 8 with the pool drops.
Here is the test with and without a flash using the hydro store drops.

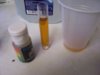
Had the pool drops avail so I checked with those since they are within range and it looked like 6 and again my tap looked like 8 with the pool drops.
Here is the test with and without a flash using the hydro store drops.



