Ammastor
Active Member
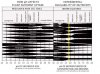
Figured I would post this chart that I have had for a long while. I am sure it has been on here before and most of you have seen it but if not this may be of help to some of you that are having issues with some of your plants. As PH ranges are critical to get right for growth, health, and higher yields.
View attachment 2888510
This chart shows at what PH different nutrients are absorbed by the little girls. Helpful for diagnosing. Especially in Soil.
What I recommend people doing is keeping a log of PH as you test it by date time and so on.
If your plants run into a problem you can refer to this chart for trouble shooting a deficiency.
OR
Use it as a guide to where your PH should be in soil or hydro
View attachment 2888510
This chart shows at what PH different nutrients are absorbed by the little girls. Helpful for diagnosing. Especially in Soil.
What I recommend people doing is keeping a log of PH as you test it by date time and so on.
If your plants run into a problem you can refer to this chart for trouble shooting a deficiency.
OR
Use it as a guide to where your PH should be in soil or hydro