elibaltutiimaidumituti
Member
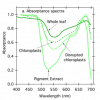
Here in this diagram you can see the differences in excitation between the two photosystems:
View attachment 4728806
From "Photobiologie" by L. O. Björn
The PSI drop @ 652nm is created by Chlorophyl-b - which mostly distributes energy towards PSII.
That basically means that 660nm reds would not be sufficient alone to create a better electron-flow (or: to do away with that PSI bottleneck).
If one wants to get the idea, one just needs to look at the suns immense inclusion of red, darkred (FR/IR) to see, why alot of LED spectra are, sort of, incomplete - or better: "in development".
Well, the new Samsung lm301h-ONE chip did muster up in that regard a bit... (also on green, but that is an entire different story...)
thank you for the tech talk! i have spent to much time studying lower frequency UV that i am behind on my studies of DR/FR/IR. there is a mystery to me still in what the fuck is IR truly...
Is it heat? Does it make heat? Some say that it is a photonic particle of light which engages by impact the molecular vibrations that initiate thermal radiation. But Nikola felt that the idea of a light particle was foolishness. So... what the fuck is IR? It’s not a wave, it’s not a ray. It’s not some wave that pretends to be a particle when we look at it. It’s not light thus cannot be a photon of light causing thermal vibrations... it is not a causal disruption of waves upon the aether fluid either... it’s something else
I already hear the electromagnetic wave responses but it’s not that if electrons are fictional...
...but mainly, i hear rumors of adding IR to your garden... this or that light has IR diodes... are people just confusing IR with FR? or is there some benefit to the plants in “our” community garden from IR exposure?
(crosses fingers, hopes to learn that IR triples quality)
How does Infrared Relate to heat?
I never understood the relationship between Infrared and Heat. Is IR emitted when heat is generated, is heat generated when IR is made, and how do the two relate?