ChiefRunningPhist
Well-Known Member
Just a quick little post on LED effeciency.
Phosphor conversion effeciencies (CE) are never over 1. They approach 1, but never exceed 1 (about 99.9%). This essentially means that photons emitted are maintained, ie the phosphor film doesn't diminish the initial photon quantity, all photons are conserved.
A white LED uses a blue pump, or a blue "base" diode to create the initial quantity of photons, and then the phosphor film layer converts all the blue photons created by the base pump to all sorts of different color photons.
Every photons' WV has a unique energy associated with it. This energy can be expressed in terms of μmol/J, or the amount of energy (J) per photon qty (μmol). A 450nm photon will have a higher and different distinct energy than a 451nm. A 646nm has its distinct energy, a 757nm photon has its distinct energy, ect ect. If the WV changes so does the energy. This energy can be easily calculated with the 119.3 conversion factor.
119.3 ÷ WV = J/μmol
WV ÷ 119.3 = μmol/J
Example:
455nm ÷ 119.3 = 3.81μmol/J
119.3 ÷ 455nm = 0.262J/μmol
445nm ÷ 119.3 = 3.73μmol/J
119.3 ÷ 445nm = 0.268μmol/J
With just a 10nm difference in base pump one can see a diminished photon effeciency of one over the other. Which would you rather have, 3.81 or 3.73?
To determine % effeciency of an LED given in μmol/J, you'd want to find the peak WV of the base pump by looking at the SPD on the datasheet. Then you'd divide the peak base pump WV by 119.3 to achieve the max possible μmol/J, and then you'd look at the data sheet for what it says it's μmol/J effeciency is.
Example:
80CRI 3500k LM301h rated @ 2.67 μmol/J
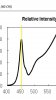
Looks like peak base pump WV is around 455nm.
455 ÷ 119.3 = 3.81μmol/J
(2.67 μmol/J) ÷ (3.18μmol/J)
=
0.70 or 70% effecient
At 70% effeciency, ~30% of consumed power would need to be calculated for heatsink needs. There will be some additional heat losses on the phosphor film due to conversion though and I'm not exactly sure what percentage of the remaining 70% will be converted to heat (maybe 5%, maybe 10%, it'll be dependepent on SPD). The farther the conversion, ie the farther away from the base pump WV, the higher the heat loss on the phosphor film.
Because the conversion factor (119.3) never changes you can easily compare WV photon effeciencies by simply comparing their WVs.
650nm ÷ 450nm = 1.44, this means that if 2 chips were present, both having equal effeciencies and power consumption rates but different WVs, ie 1 is a 450nm chip and 1 is a 650nm chip, the 650nm chip will produce 1.44x more photons than the 450nm chip (144%).
Phosphor conversion effeciencies (CE) are never over 1. They approach 1, but never exceed 1 (about 99.9%). This essentially means that photons emitted are maintained, ie the phosphor film doesn't diminish the initial photon quantity, all photons are conserved.
A white LED uses a blue pump, or a blue "base" diode to create the initial quantity of photons, and then the phosphor film layer converts all the blue photons created by the base pump to all sorts of different color photons.
Every photons' WV has a unique energy associated with it. This energy can be expressed in terms of μmol/J, or the amount of energy (J) per photon qty (μmol). A 450nm photon will have a higher and different distinct energy than a 451nm. A 646nm has its distinct energy, a 757nm photon has its distinct energy, ect ect. If the WV changes so does the energy. This energy can be easily calculated with the 119.3 conversion factor.
119.3 ÷ WV = J/μmol
WV ÷ 119.3 = μmol/J
Example:
455nm ÷ 119.3 = 3.81μmol/J
119.3 ÷ 455nm = 0.262J/μmol
445nm ÷ 119.3 = 3.73μmol/J
119.3 ÷ 445nm = 0.268μmol/J
With just a 10nm difference in base pump one can see a diminished photon effeciency of one over the other. Which would you rather have, 3.81 or 3.73?
To determine % effeciency of an LED given in μmol/J, you'd want to find the peak WV of the base pump by looking at the SPD on the datasheet. Then you'd divide the peak base pump WV by 119.3 to achieve the max possible μmol/J, and then you'd look at the data sheet for what it says it's μmol/J effeciency is.
Example:
80CRI 3500k LM301h rated @ 2.67 μmol/J

Looks like peak base pump WV is around 455nm.
455 ÷ 119.3 = 3.81μmol/J
(2.67 μmol/J) ÷ (3.18μmol/J)
=
0.70 or 70% effecient
At 70% effeciency, ~30% of consumed power would need to be calculated for heatsink needs. There will be some additional heat losses on the phosphor film due to conversion though and I'm not exactly sure what percentage of the remaining 70% will be converted to heat (maybe 5%, maybe 10%, it'll be dependepent on SPD). The farther the conversion, ie the farther away from the base pump WV, the higher the heat loss on the phosphor film.
Because the conversion factor (119.3) never changes you can easily compare WV photon effeciencies by simply comparing their WVs.
650nm ÷ 450nm = 1.44, this means that if 2 chips were present, both having equal effeciencies and power consumption rates but different WVs, ie 1 is a 450nm chip and 1 is a 650nm chip, the 650nm chip will produce 1.44x more photons than the 450nm chip (144%).
Attachments
Last edited: