That could explain why I still get lockout even when PH'ing. I don't own a TDS, but I know my tap water is SUPER hard, high TDS.. My Hydro guy was telling me that the water just gets too congested for the nutes and cal/mag to work correctly.. Kind of like a traffic jam..
Google Mulder's chart and nutrient antagonism.
Controlling PPM doesn't really apply to soil, but as someone pointed out it can still be useful to give an indication. If you don't overfertilize there's usually no need to even measure ppm though.
imo, PH doesn't matter in soil; however, i'm in the process now of determining if that original conclusion based on many months of studying and growing (with and without PH measurements) is correct. there is one plant that is definitely showing PH problems though while everything else is fine. can you elaborate please? specifically regarding your definition of fertilizing the soil properly. thank you.s!
Whether pH matters in soil is not an opinion, it either does or it doesn't. And it sure does, but it's a wide range which in combination with normal fluctuations and variation of ph level throughout the soil makes it unnecessary to control. It often leads to more problems. If you want pH control grow hydro. At most controlling it matters if the tap water is too high by itself, or after miss-fertilizing (giving too much of everything or too much of one or more elements) but if the tap is high and the ph drops from over-fertilizing one can simply give water only for a while. The uptake of some elements increases pH, others cause it to drop.
<-not a dick pic mods.
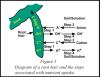
Note the H+ and the OH- the plant releases to exchange with a useful element. The pH is a measure of the molar concentration of hydrogen ions.
A typical example is high P nutrients. P is an anion (negative charged) so to take it up the plant releases a positive hydrogen ions (H+). The concentration of H+ is what the pH level determines, the more H+, the lower the pH.
The nutrient elements, the ions (cations and anions) move throughout the pot, partly by a process called diffusion, partly by water uptake/movement. This results in different concentrations of nutrients throughout a soil container. That in turn (because certain ph values are better for take up of certain elements, which influence pH) leads to different pH values throughout the pot. Which in turn, as I mentioned earlier, is actually an advantage, there's always a spot in the pot where the plant can take up zinc, and a spot where it can take up N easily etc.etc.
By trying to steer the pH you take over the job from the plant and that doesn't always lead to a better situation (often not...). Also once you start controlling you generally have to continue doing so.
The pH value, in soil too, is important because certain elements are easier to take up within a certain pH range. It differs per element, there's not 1 specific optimal pH value, it's a range that includes all elements. A common mistake hydrogrowers make is keeping the ph strict on 5.8 while it's good when it fluctuates a little between low (good for micro uptake) and high (good for macro uptake).

